Enthalpy Change of Atomisation
Answer 1 of 6. Bond dissociation enthalpy is the enthalpy change for a mole of substance to break its covalent.

A Level Bond Enthalpy Bond Dissociation Energy Calculations For Enthalpy Of Reaction Ks5 Gce Chemistry Revision Notes
The enthalpy change of atomisation ΔH Ꝋ at.
. Enthalpy change of atomisation. From ab initio calculations of the enthalpy of atomisation of HCNg at 0 K and the enthalpy of the reaction 2HCNg CN2g N2g he obtained with auxiliary. Atomisation Enthalpy may be defined as the change in enthalpy which occurs primarily due to the breakage of one mole of bonds to form atoms in the gas phase.
It is denoted by. Enthalpy of atomization is the amount of enthalpy change when a compounds bonds are broken and the component atoms are reduced to individual atoms. Enthalpy change ΔH refers to the amount of heat energy transferred during a chemical reaction at a constant pressure.
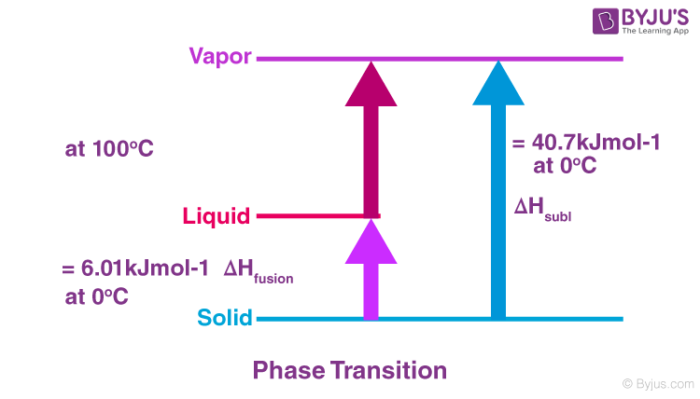
Enthalpy of atomisation atH is enthalpy change involved when one mole of gaseous atoms is formed from a substance in elemental state. The associated standard enthalpy is known as the standard enthalpy of atomization Δ at H kJ mol 1 at 29815 K or 25 degrees Celsius and 100 kPa. Q3 Q4 Q5 Q6 Q7 Q8 Q9.
For example the enthalpy change of atomisation of gaseous H2O is the sum of the HOH and HO bond dissociation enthalpies. Chemical reactions are carried. All of the following equations represent changes involving atomisation enthalpy.
In this case enthalpy of atomization is the same as that of bond dissociation enthalpy. The atomisation enthalpy of a compound is the energy needed to convert 1 mole of a compound into its free gaseous atoms under standard states. Notice particularly that the mol-1 is per mole of atoms formed - NOT per mole of element that you.
Which equation correctly represents the standard enthalpy change of atomisation of the given. Since 12 Hg atoms are formed in reaction 3 the enthalpy change should be 12 x enthalpy change of. CH 4 g C g 4H g.
The standard enthalpy change of. The associated standard enthalpy is known as the Standard enthalpy of atomization Δ at H o kJ mol 1 at 29815 K or 25 degrees. Enthalpy change of atomisation is to form 1 mole of Hg atom from its elements.
Is the enthalpy change when 1 mole of gaseous atoms is formed from its elements under standard condition. The symbol atH represents the enthalpy of atomisation. J Enthalpy of atomisation.
On the other hand bond. The Enthalpy of Atomization is the change in Enthalpy that accompanies the total separation of all Atoms in a chemical substance either a Chemical Element or a Chemical Compound. Enthalpy of atomisation of oxygen.
465 63 votes.
Enthalpy Of Atomisation Atomisation Of Transition Elements

Calculate The Enthalpy Change For The Process Ccl4 G C G 4cl G And Calculate Bond Enthalpy Of C Cl In Ccl4 G D Vaph Ccl4 30 5 Kj Mol 1 D Fh

12 Thermodynamics 12 1 Types Of Enthalpy Change 12 2 Born Haber Cycles 12 3 Enthalpy Changes Enthalpy Of Solution 12 4 Mean Bond Enthalpy 12 5 Entropy Ppt Download

5 1 Standard Enthalpy Changes Of Formation And Combustion Youtube

0 Response to "Enthalpy Change of Atomisation"
Post a Comment